COVID-19
COVID-19
Decision-Making During the COVID-19 PandemicBackground
COVID-19 pandemics has challenged emergency response systems worldwide, with widespread reports of essential services breakdown and collapse of health care structure. Testing capacity is also problematic in several countries, where diagnosis demand outnumbers available local testing capacity.
Methods
A Naïve-Bayes model for machine learning is proposed for handling different scarcity scenarios. Hemogram result data was used to predict qRT-PCR results in situations where the latter was not performed, or results are not yet available. Adjusts in assumed prior probabilities allow fine-tuning of the model, according to actual prediction context. The results can be used for screening of patients to be tested in a scenario where few qRT-PCR or IgM/IgG tests are available.
Additional Information
Instructions
- All variables must be filled in.
- The units must be respected.
- Prior choice: select the prior probability of Covid-19 infection. Adjust for each patient according to symptoms and contamination risk. We suggest the use of 0.5 (when no information is available, meaning there is an equal probability of positive or negative result before using hemogram data) or disease prevalence in local population, when available. Keep in mind that a 0.5 prior is very conservative and while it correctly identifies most positive cases, it also leads to a higher number of false positive results.
- This tool is based on blood alterations causes by Covid-19 in symptomatic patients. It is possible that other inflammatory or Infectious processes also cause similar alterations.
- The results should only be used for screening patients who will perform qRT-PCR or IgM/IgG antibody detection tests.
- The results of this tool do not replace any tests such as RT-PCR or IgG/IgM antibody detection.
Contact Us
Dr. Márcio Dorn - UFRGS
Dr. Clarice Sampaio Alho - PUCRS
Dr. Eduardo Avila - INCT/PF
Dr. Alessandro Kahmann - FURG
Complete Blood Count
Probability Density Function
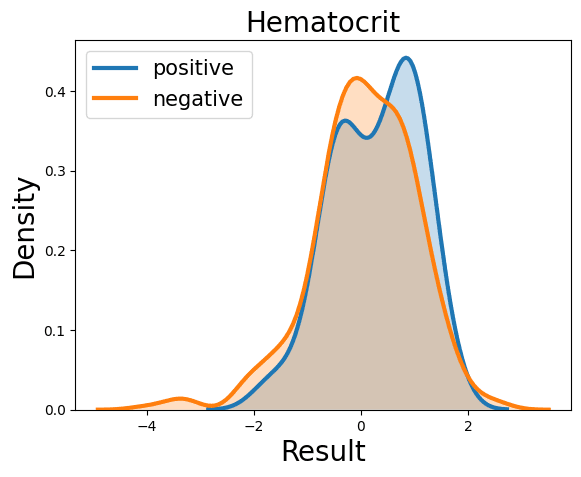
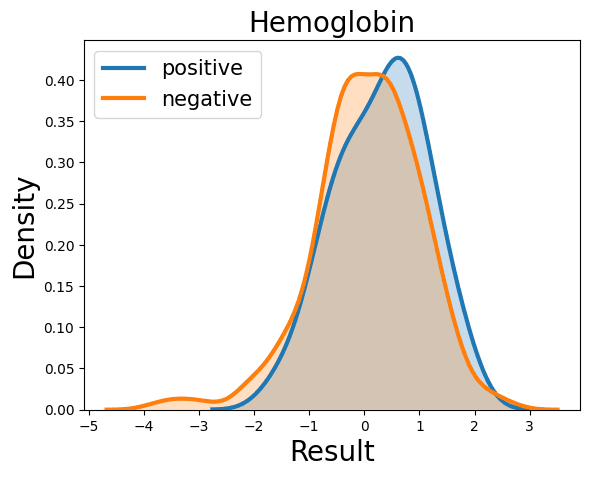
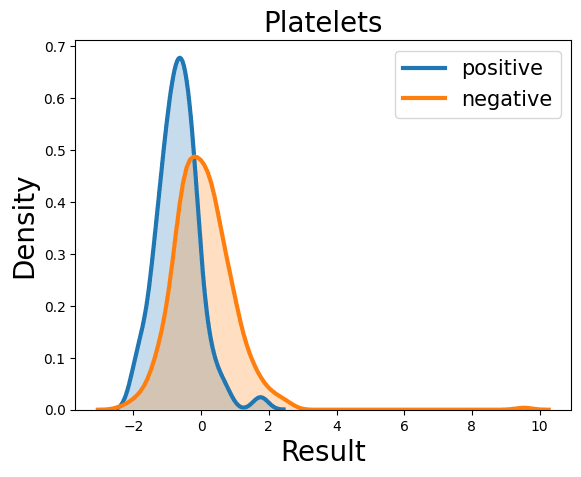
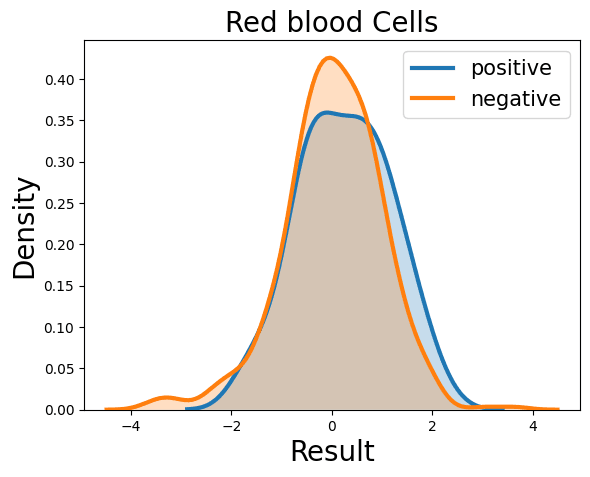
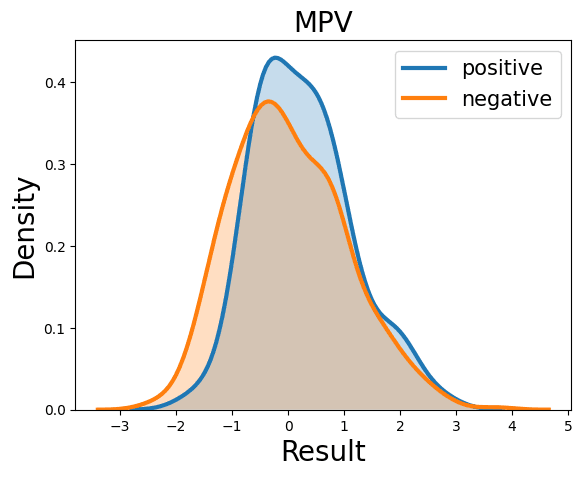
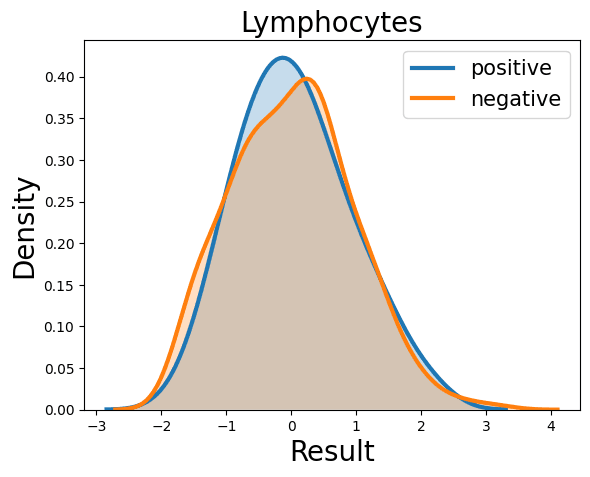
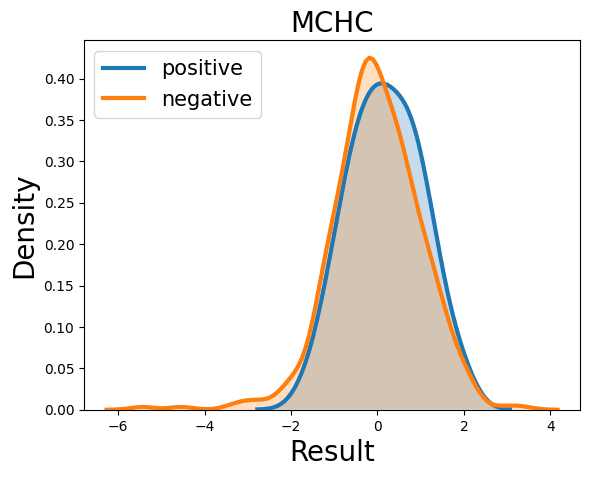
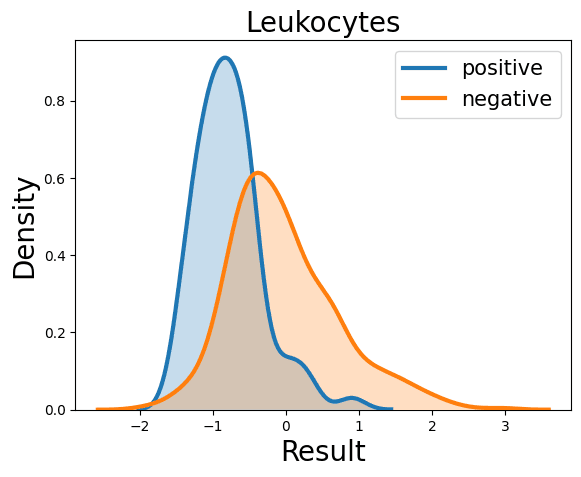
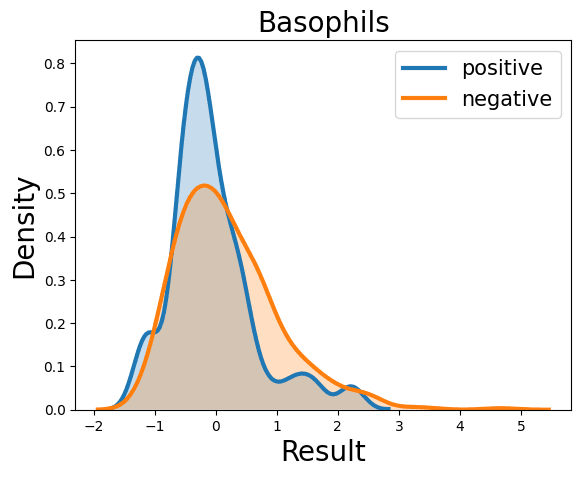
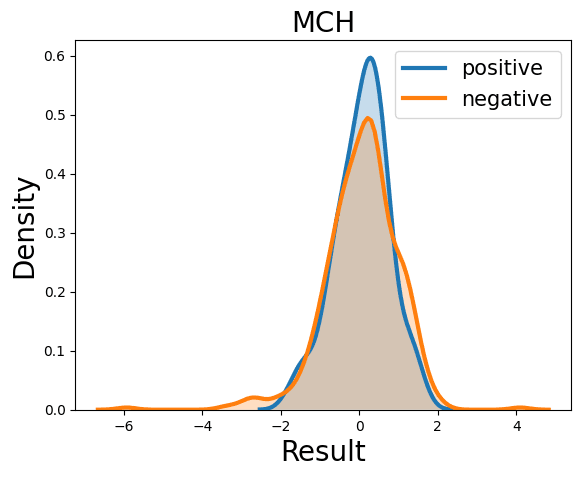
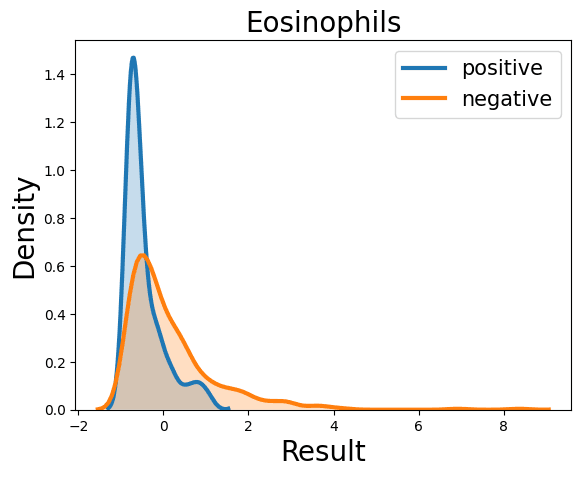
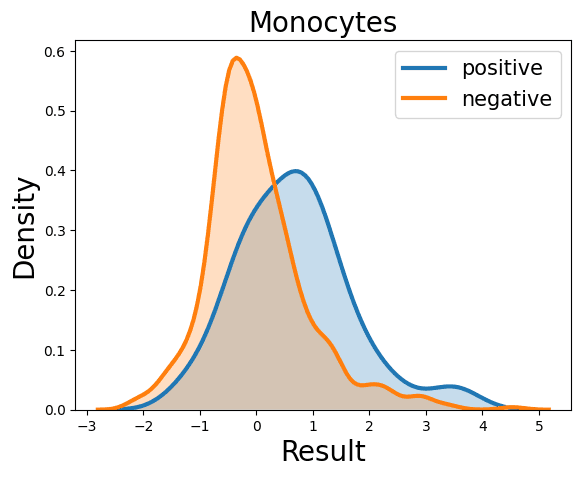
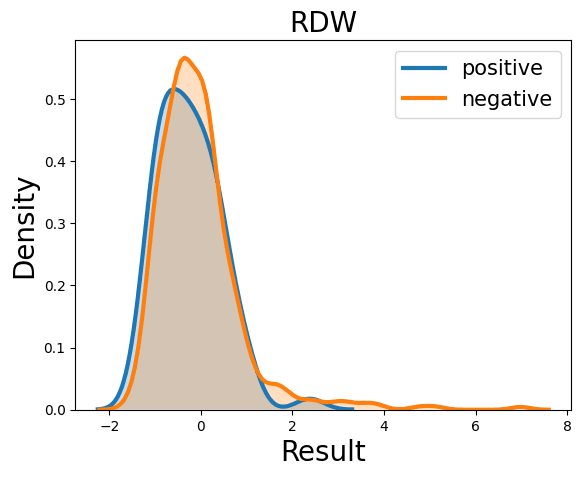
References
- Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis HENRY, B. M.; DE OLIVEIRA, M. H. S.; BENOIT, S.; PLEBANI, M.; LIPPI, G. Clinical Chemistry and Laboratory Medicine (CCLM), v. 58, p. 1021-1028, 2020.
- Hematological findings and complications of COVID-19 TERPOS, E.; NTANASIS-STATHOPOULOS, I.; ELALAMY, I.; KASTRITIS, E.; SERGENTANIS, T. N.; POLITOU, M.; PSALTOPOULOU, T.; GEROTZIAFAS, G.; DIMOPOULOS, M. A. American Journal of Hematology, v. 96, p. 834-847, 2020.
- Laboratory abnormalities in patients with COVID-2019 infection LIPPI, G.; PLEBANI, M. Clinical Chemistry and Laboratory Medicine (CCLM), v. 58, p. 1131-1134, 2020.
- Prominent changes in blood coagulation of patients with SARS-CoV-2 infection HAN, H.; YANG, L.; LIU, R.; LIU, F.; WU, K.; LI, J.; LIU, X.; ZHU, C. Clinical Chemistry and Laboratory Medicine (CCLM), v. 58, p. 1116-1120, 2020.
- Routine blood tests as a potential diagnostic tool for COVID-19 FERRARI, D.; MOTTA, A.; STROLLO, M.; BANFI, G.; LOCATELLI, M. Clinical Chemistry and Laboratory Medicine (CCLM), v. 58, p. 1095-1099, 2020.